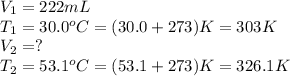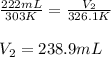Chemistry, 07.05.2020 00:09 TwentyOnePawPrints21
A balloon at 30.0°C has a volume of 222 mL. If the temperature is
increased to 53.1°C and the pressure remains constant, what will the new volume be, in ml?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
A balloon at 30.0°C has a volume of 222 mL. If the temperature is
increased to 53.1°C and the p...
increased to 53.1°C and the p...
Questions


Mathematics, 24.06.2020 06:01



Mathematics, 24.06.2020 06:01




Mathematics, 24.06.2020 06:01


Mathematics, 24.06.2020 06:01

Mathematics, 24.06.2020 06:01


Mathematics, 24.06.2020 06:01


Mathematics, 24.06.2020 06:01

World Languages, 24.06.2020 06:01



Mathematics, 24.06.2020 06:01

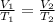
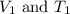 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.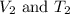 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.