

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1


You know the right answer?
A 75.0-mLmL volume of 0.200 MM NH3NH3 (Kb=1.8×10−5Kb=1.8×10−5) is titrated with 0.500 MM HNO3HNO3. C...
Questions

Mathematics, 04.09.2020 14:01

History, 04.09.2020 14:01

English, 04.09.2020 14:01

Mathematics, 04.09.2020 14:01

Chemistry, 04.09.2020 14:01

Health, 04.09.2020 14:01



Mathematics, 04.09.2020 14:01

Social Studies, 04.09.2020 14:01

Health, 04.09.2020 14:01








Law, 04.09.2020 14:01

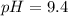
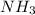 is
is 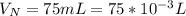
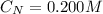
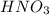 is
is 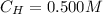
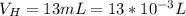
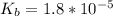
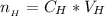
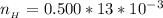
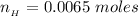
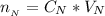
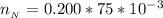
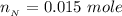
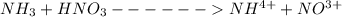
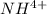
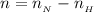
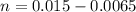
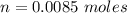
![pH = pK_a + log [\frac{NH_3}{NH^{4+}} ]](/tpl/images/0650/7966/5c1fa.png)
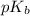 is mathematically represented as
is mathematically represented as 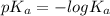
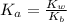
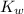 is the ionization constant of
is the ionization constant of 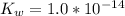
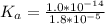
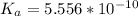
![pH = -log K_a + log [\frac{NH_3}{NH^{4+}} ]](/tpl/images/0650/7966/57494.png)
![pH = log [\frac{\frac{NH_3}{NH^{4+}} }{K_a} ]](/tpl/images/0650/7966/7943c.png)
![pH = log [\frac{\frac{0.0085}{0.0065} }{5.556*10^{-10}} ]](/tpl/images/0650/7966/d002a.png)


