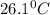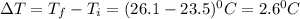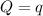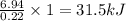Chemistry, 07.05.2020 05:16 rosepetals2938
The energy content of food is typically determined using a bomb calorimeter. Consider the combustion of a 0.22-g sample of butter in a bomb calorimeter having a heat capacity of 2.67 kJ/°C. If the temperature of the calorimeter increases from 23.5°C to 26.1°C, calculate the energy of combustion per gram of butter. Energy of combustion = kJ/g

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1
You know the right answer?
The energy content of food is typically determined using a bomb calorimeter. Consider the combustion...
Questions


Mathematics, 24.04.2020 11:23

Social Studies, 24.04.2020 11:24

Mathematics, 24.04.2020 11:24


Mathematics, 24.04.2020 11:24



English, 24.04.2020 11:49


Biology, 24.04.2020 11:49

Physics, 24.04.2020 11:49

Biology, 24.04.2020 11:49

English, 24.04.2020 11:49


Mathematics, 24.04.2020 11:49


Geography, 24.04.2020 11:49

Mathematics, 24.04.2020 11:49

Mathematics, 24.04.2020 11:50

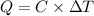
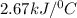
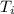 =
= 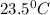
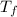 =
=