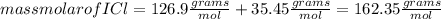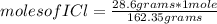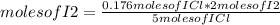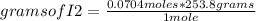Chemistry, 06.11.2019 07:31 shayravirgen30
Iodine chloride, icl, can be made by the following reaction between iodine, i2, potassium iodate, kio3, and hydrochloric acid.
2 i2 + kio3 + 6 hcl > 5 icl + kcl + 3 h2o
calculate how many grams of iodine are needed to prepare 28.6 grams of icl by this reaction.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:50
What does standard deviation reveal about data? a. the average of all the data points b. which of the data points is most reliable c. how spread out the data points are d. the percent error included in the data
Answers: 2

Chemistry, 21.06.2019 16:30
What is the force of attraction between the particles in a salt crystal
Answers: 2

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Iodine chloride, icl, can be made by the following reaction between iodine, i2, potassium iodate, ki...
Questions

Mathematics, 23.11.2020 06:00



Chemistry, 23.11.2020 06:00


Mathematics, 23.11.2020 06:00


Mathematics, 23.11.2020 06:00

English, 23.11.2020 06:00



Mathematics, 23.11.2020 06:00

Social Studies, 23.11.2020 06:00


Computers and Technology, 23.11.2020 06:00

Chemistry, 23.11.2020 06:00

English, 23.11.2020 06:00

English, 23.11.2020 06:00

English, 23.11.2020 06:00

Geography, 23.11.2020 06:00