
Which of the following best describes what causes water molecules to exhibit properties such as cohesive and adhesive behavior?
a. water molecules are polar, having partially positive and partially negative ends.
b. water molecules have both a hydrophobic end and a hydrophilic end.
c. water molecules are capable of dissolving both polar and nonpolar substances.
d. water molecules have a high molecular weight, making them very dense.

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
Which of the following best describes what causes water molecules to exhibit properties such as cohe...
Questions






Biology, 16.09.2019 17:40



English, 16.09.2019 17:50

English, 16.09.2019 17:50

Mathematics, 16.09.2019 17:50


Mathematics, 16.09.2019 17:50



Mathematics, 16.09.2019 17:50

Social Studies, 16.09.2019 17:50

Mathematics, 16.09.2019 17:50


Mathematics, 16.09.2019 17:50

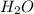 . It is a polar covalent molecule and in each O-H bond, oxygen has a partial negative charge and hydrogen a partial positive charge. Water molecules have strong cohesive forces as they can form intermolecular hydrogen bonds. Water molecules can also show strong adhesive forces with the walls of the container it holds due to the polar nature of the molecules.
. It is a polar covalent molecule and in each O-H bond, oxygen has a partial negative charge and hydrogen a partial positive charge. Water molecules have strong cohesive forces as they can form intermolecular hydrogen bonds. Water molecules can also show strong adhesive forces with the walls of the container it holds due to the polar nature of the molecules. 

