
Chemistry, 10.05.2020 09:57 tiwaribianca475
Double and triple bonds form because:
a. one of the atoms in the molecule has more than eight valence electrons
b. single covalent bonds do not give all of the atoms in the molecule eight valence electrons
c. the ions involved have charges larger than one.
d. there is at least one hydrogen atom involved in the bond.
e. the atoms involved have high electronegativities.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

You know the right answer?
Double and triple bonds form because:
a. one of the atoms in the molecule has more than...
a. one of the atoms in the molecule has more than...
Questions


Mathematics, 26.05.2021 01:50




Mathematics, 26.05.2021 01:50

Chemistry, 26.05.2021 01:50




Biology, 26.05.2021 01:50



Biology, 26.05.2021 01:50

Biology, 26.05.2021 01:50


Mathematics, 26.05.2021 01:50

Mathematics, 26.05.2021 01:50

Mathematics, 26.05.2021 01:50

Mathematics, 26.05.2021 01:50

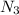 . Nitrogen has 5 valence electrons each, meaning it needs 3 more electrons to fill its outer shell. Nitrogen can fill its outer shell by simply forming 3 covalent bonds with another Nitrogen atom.
. Nitrogen has 5 valence electrons each, meaning it needs 3 more electrons to fill its outer shell. Nitrogen can fill its outer shell by simply forming 3 covalent bonds with another Nitrogen atom.

