A ball has a volume of 6.35 liters and is at a temperature of
27.0°C. A pressure gauge attache...

Chemistry, 10.05.2020 06:57 ShugarLove4363
A ball has a volume of 6.35 liters and is at a temperature of
27.0°C. A pressure gauge attached to the ball reads 0.45
atmosphere. The atmospheric pressure is 1.00 atmosphere.
Calculate the absolute pressure inside the ball and the
amount of moles of air it contains. (First, find the absolute
pressure and then use that to find the moles using the ideal
gas law. Remember to use the correct ideal gas constant and
to convert from celsius to kelvin.)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2


Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2

Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1
You know the right answer?
Questions

Mathematics, 27.01.2021 01:00


Mathematics, 27.01.2021 01:00


Mathematics, 27.01.2021 01:00


Biology, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00


Mathematics, 27.01.2021 01:00


Arts, 27.01.2021 01:00


Mathematics, 27.01.2021 01:00

Social Studies, 27.01.2021 01:00


Mathematics, 27.01.2021 01:00

Business, 27.01.2021 01:00

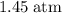 .Number of moles of air particles inside the ball, by the ideal gas law: approximately
.Number of moles of air particles inside the ball, by the ideal gas law: approximately 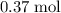 .
. 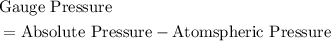 .
.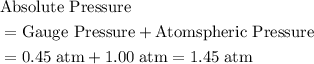 .
.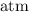 for pressure and
for pressure and 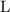 for volume.
for volume.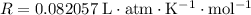 .
.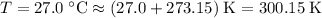 .
.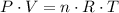 (after rearranging) to find the number of moles of gas particles in this ball:
(after rearranging) to find the number of moles of gas particles in this ball: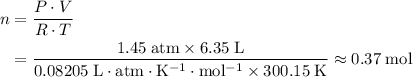 .
.

