
Chemistry, 13.05.2020 09:57 hannahrasco4051
The activation energy for proline isomerization of a peptide depends on the identity of the preceding residue and obeys Arrhenius rate behavior. Experiments are conducted on the isomerization of an alanine- proline peptide. At 25°C (298 K) the observed rate constant is 0.05 sec–1 and the value of EA is calculated to be 60 kJ•mol–1. Similar measurements are performed on a phenylalanine-proline peptide at 25°C, with a measured rate constant of 0.005 sec–1. Assuming an identical preexponential factor as the alanine-proline peptide, what is the activation energy for this peptide (kJ/mol)?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
You know the right answer?
The activation energy for proline isomerization of a peptide depends on the identity of the precedin...
Questions





Mathematics, 29.11.2019 04:31

Health, 29.11.2019 04:31



SAT, 29.11.2019 04:31

Chemistry, 29.11.2019 04:31










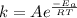 , where k is rate constant, A is pre-exponential factor,
, where k is rate constant, A is pre-exponential factor, 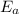 is activation energy, R is gas constant and T is temperature in kelvin scale.
is activation energy, R is gas constant and T is temperature in kelvin scale.![\frac{k_{ala-pro}}{k_{phe-pro}}=e^\frac{[E_{a}^{phe-pro}-E_{a}^{ala-pro}]}{RT}](/tpl/images/0653/2427/f6d0c.png)
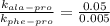 , T = 298 K , R = 8.314 J/(mol.K) and
, T = 298 K , R = 8.314 J/(mol.K) and 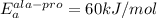
![\frac{0.05}{0.005}=e^{\frac{[E_{a}^{phe-pro}-(60000J/mol)]}{8.314J.mol^{-1}.K^{-1}\times 298K}}](/tpl/images/0653/2427/26a26.png)

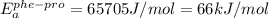 (rounded off to two significant digit)
(rounded off to two significant digit)

