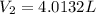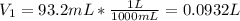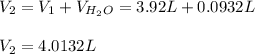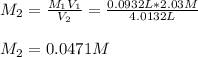is diluted with 3.92 L of water.


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
93.2 mL of a 2.03 M potassium fluoride (KF) solution
is diluted with 3.92 L of water.
is diluted with 3.92 L of water.
Questions

Mathematics, 22.04.2021 15:50

Mathematics, 22.04.2021 15:50


Mathematics, 22.04.2021 15:50

Mathematics, 22.04.2021 15:50





Mathematics, 22.04.2021 15:50


Mathematics, 22.04.2021 15:50

Computers and Technology, 22.04.2021 15:50

English, 22.04.2021 15:50



Mathematics, 22.04.2021 15:50



Geography, 22.04.2021 15:50