Question Completion Status:
[07.03)
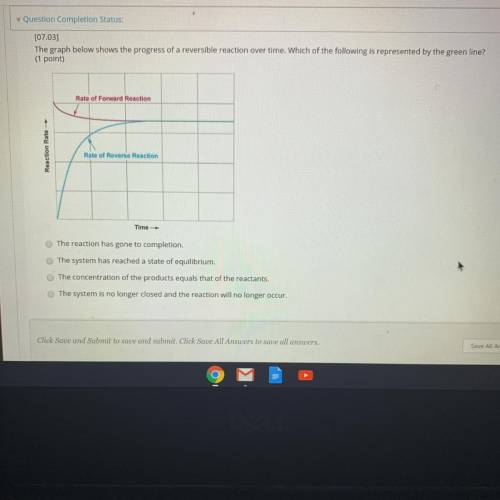
The graph below shows the progress of a reversible r...

Question Completion Status:
[07.03)
The graph below shows the progress of a reversible reaction over time. Which of the following is represented by the green line?
(1 point)
Rate of Forward Reaction
Reaction Rate -
Rate of Reverse Reaction
Time
The reaction has gone to completion.
The system has reached a state of equilibrium.
The concentration of the products equals that of the reactants,
The system is no longer closed and the reaction will no longer occur.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
Questions


Mathematics, 14.11.2020 08:30


Health, 14.11.2020 08:30




English, 14.11.2020 08:30



Mathematics, 14.11.2020 08:30




Chemistry, 14.11.2020 08:30

Mathematics, 14.11.2020 08:30

Social Studies, 14.11.2020 08:30

History, 14.11.2020 08:30

Biology, 14.11.2020 08:30

Chemistry, 14.11.2020 08:30



