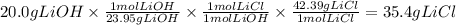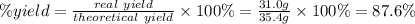Chemistry, 19.05.2020 02:15 jluckie080117
A reaction starts with 20.0 g of lithium hydroxide (LiOH) and actually produces 31.0 g of lithium chloride (LiCl), what is the percent yield? (Hint: First calculate the theoretical yield of lithium chloride (LiCl))
64.5%
88.6%
81.5%
92.8%


Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1
You know the right answer?
A reaction starts with 20.0 g of lithium hydroxide (LiOH) and actually produces 31.0 g of lithium ch...
Questions





Mathematics, 10.05.2021 20:00

Mathematics, 10.05.2021 20:00

Mathematics, 10.05.2021 20:00


Physics, 10.05.2021 20:00

Mathematics, 10.05.2021 20:00

Chemistry, 10.05.2021 20:00

Computers and Technology, 10.05.2021 20:00





Mathematics, 10.05.2021 20:00