Given the following equation: Ca(OH)2 + 2HNO3 --> 2 H2O
+ Ca(NO3)2
How many moles of...

Chemistry, 19.05.2020 02:10 cranfordjacori
Given the following equation: Ca(OH)2 + 2HNO3 --> 2 H2O
+ Ca(NO3)2
How many moles of water are produced when 2.27
moles of calcium hydroxide reacts with excess nitric
acid?
Please include your units and round to two decimal places.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1

Chemistry, 23.06.2019 15:30
Which term defines a type of oxygen that forms a protective layer miles above the earth a. fossil fuel b. smog c. pollution d. ozone
Answers: 2
You know the right answer?
Questions

Mathematics, 16.10.2020 14:01

Social Studies, 16.10.2020 14:01




Mathematics, 16.10.2020 14:01

World Languages, 16.10.2020 14:01



English, 16.10.2020 14:01

Physics, 16.10.2020 14:01

Social Studies, 16.10.2020 14:01


Mathematics, 16.10.2020 14:01


Mathematics, 16.10.2020 14:01

Mathematics, 16.10.2020 14:01


Health, 16.10.2020 14:01

Health, 16.10.2020 14:01

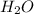 will be produced.
will be produced.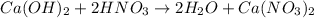
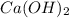 produce = 2 moles of
produce = 2 moles of 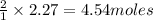 of
of 

