
Chemistry, 19.05.2020 15:58 cameronrandom00
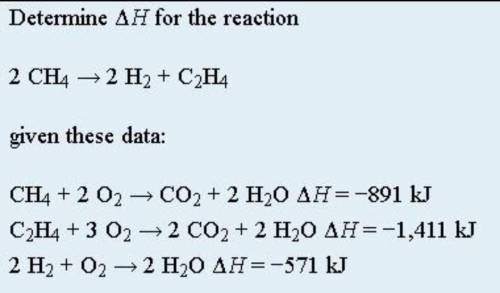
Hess’s law is very powerful. It allows us to combine equations to generate new chemical reactions whose enthalpy changes can be calculated, rather than directly measured. Besides that, Hess’s law states that when chemical equations are combined algebraically, their enthalpies can be combined in exactly the same way. Below points are to be taken as well: 1) If a chemical reaction is reversed, the sign on ΔH is changed; 2) If a multiple of a chemical reaction is taken, the same multiple of the ΔH is taken as well. As attached is an example of Hess Law problem solving question. By using Hess law , combine the equation algebraically and determine the enthaphy change of the reaction.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
Hess’s law is very powerful. It allows us to combine equations to generate new chemical reactions wh...
Questions

Biology, 01.12.2019 18:31



Biology, 01.12.2019 18:31





Mathematics, 01.12.2019 18:31


History, 01.12.2019 18:31

Mathematics, 01.12.2019 18:31

History, 01.12.2019 18:31

Social Studies, 01.12.2019 18:31


Biology, 01.12.2019 18:31

Mathematics, 01.12.2019 18:31





