Amning At Home -Chemisu
Use the specific heat values to answer the
following questions.<...

Chemistry, 19.05.2020 16:09 lizzyhearts
Amning At Home -Chemisu
Use the specific heat values to answer the
following questions.
Cp (J/g•°C)
4.18
Which of the following has the smallest heat
capacity?
2.03
2.08
0.450
RETRY
Substance
H2O(1)
H2O(s)
H2O(g)
Fe(s)
Al(s)
Cu(s)
Sn(s)
Pb(s)
Au(s)
Hg(1)
0.897
0.385
0.227
0.129
0.129
0.140
Intro

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
Questions







Business, 08.04.2020 22:01

Mathematics, 08.04.2020 22:01



Mathematics, 08.04.2020 22:01







Mathematics, 08.04.2020 22:01

Mathematics, 08.04.2020 22:01

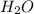 (l)
(l)

