
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a 500. mL flask with 3.7 atm of sulfur dioxide gas and 2.3 atm of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 2.2 atm. Calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2


Chemistry, 23.06.2019 14:20
Identificaa 5 características que comparten las guacamayas y dos caracteristicas que las hagan diferentes
Answers: 1
You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions

Social Studies, 17.12.2020 02:20


English, 17.12.2020 02:20

Biology, 17.12.2020 02:20



Mathematics, 17.12.2020 02:20



English, 17.12.2020 02:20


Mathematics, 17.12.2020 02:20

Mathematics, 17.12.2020 02:20



SAT, 17.12.2020 02:20



Mathematics, 17.12.2020 02:20

Mathematics, 17.12.2020 02:20

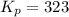
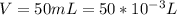
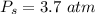
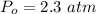
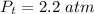
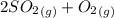 ⇄
⇄ 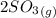
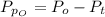
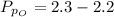
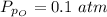
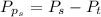
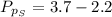
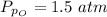
![K_p = \frac{[P_t]^2}{[P_p__{o}} ]^2 [P_p__{s}}]}](/tpl/images/0656/7414/8f8eb.png)
![K_p = \frac{[2.2]^2}{[ 0.1 ]^2 [{ 1.5}]}](/tpl/images/0656/7414/2f87d.png)


