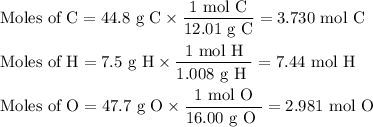Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

You know the right answer?
The sugar deoxyribose is an important component of DNA. Deoxyribose is 44.8% 7.5% H, and 47.7% O by...
Questions


Social Studies, 04.07.2019 16:30


Mathematics, 04.07.2019 16:30

Mathematics, 04.07.2019 16:30

History, 04.07.2019 16:30



Health, 04.07.2019 16:30


Mathematics, 04.07.2019 16:30

Mathematics, 04.07.2019 16:30


History, 04.07.2019 16:30


Mathematics, 04.07.2019 16:30


Mathematics, 04.07.2019 16:30

Mathematics, 04.07.2019 16:30

History, 04.07.2019 16:30