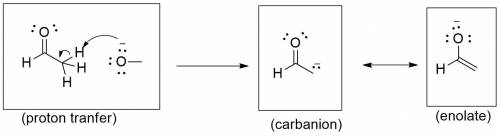
A proton transfer reaction can occur when an aldehyde is placed in strong base, such as an alkoxide ion, producing an alcohol and a charged conjugate base that is resonance stabilized. In the left box, draw the curved arrows for the proton transfer. In the middle and right boxes, draw the two structures for the resonance-stabilized product as noted in the box-specific directions. Be sure to include all lone pairs and nonzero formal charges.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3


Chemistry, 23.06.2019 06:30
How can the number of core electrons be determined from the periodic table
Answers: 1
You know the right answer?
A proton transfer reaction can occur when an aldehyde is placed in strong base, such as an alkoxide...
Questions



Geography, 09.12.2019 20:31


Chemistry, 09.12.2019 20:31


English, 09.12.2019 20:31

Mathematics, 09.12.2019 20:31

Mathematics, 09.12.2019 20:31

Mathematics, 09.12.2019 20:31

Mathematics, 09.12.2019 20:31


Mathematics, 09.12.2019 20:31


Mathematics, 09.12.2019 20:31

Mathematics, 09.12.2019 20:31


Chemistry, 09.12.2019 20:31

Biology, 09.12.2019 20:31

Mathematics, 09.12.2019 20:31