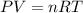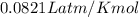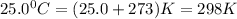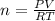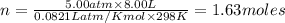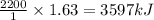Chemistry, 21.05.2020 07:59 kprincess16r
Find the heat produced from an 8.00 L cylinder of propane gas under 5.00 atm at 25.0 oC, if one mole of propane can produce 2220 kJ.
A. 4290 kJ
B. 0.0289 kJ
C. 877 kJ
D. 1.63 kJ
E. 5420 kJ
F. 1750 kJ
G. 8440 kJ
H. 1360 kJ
I. 37.2 kJ
J. 630 kJ
K. 266 kJ
L. 645 kJ
M. 2420 kJ
N. 7.36 x 10-4 kJ

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

You know the right answer?
Find the heat produced from an 8.00 L cylinder of propane gas under 5.00 atm at 25.0 oC, if one mole...
Questions

Mathematics, 22.10.2020 07:01



Mathematics, 22.10.2020 07:01

History, 22.10.2020 07:01


Mathematics, 22.10.2020 07:01

Mathematics, 22.10.2020 07:01

Mathematics, 22.10.2020 07:01

Mathematics, 22.10.2020 07:01

Social Studies, 22.10.2020 07:01


Mathematics, 22.10.2020 07:01

Mathematics, 22.10.2020 07:01

Chemistry, 22.10.2020 07:01

Physics, 22.10.2020 07:01

Mathematics, 22.10.2020 07:01

Biology, 22.10.2020 07:01

Biology, 22.10.2020 07:01