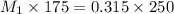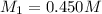Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

You know the right answer?
Water was added to 175 mL of a KOH solution until the volume was 250 mL and the molarity was 0.315 M...
Questions

Mathematics, 04.11.2020 23:40

Physics, 04.11.2020 23:40

Mathematics, 04.11.2020 23:40

History, 04.11.2020 23:40





Mathematics, 04.11.2020 23:40







History, 04.11.2020 23:40




Social Studies, 04.11.2020 23:40

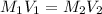
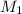 = Molarity of concentrated KOH solution = ?
= Molarity of concentrated KOH solution = ?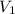 = volume of concentrated KOH solution = 175 ml
= volume of concentrated KOH solution = 175 ml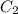 = concentration of diluted KOH solution= 0.315 M
= concentration of diluted KOH solution= 0.315 M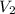 = volume of diluted KOH solution= 250 ml
= volume of diluted KOH solution= 250 ml