answer to the nearest whole number.

Chemistry, 21.05.2020 19:59 autumperry3599
Calculate AGrxn for this equation, rounding your
answer to the nearest whole number.
CaCO3(s)– Cao(s) + CO2(g)
AGf. Cacoa = -1,128.76 kJ/mol
AGf, Cao = -604.17 kJ/mol
AGT, CO, = -394.4 kJ/mol
AGrx = what

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
Calculate AGrxn for this equation, rounding your
answer to the nearest whole number.
answer to the nearest whole number.
Questions

Mathematics, 01.10.2021 01:00




Arts, 01.10.2021 01:00


Biology, 01.10.2021 01:00






Mathematics, 01.10.2021 01:00

Mathematics, 01.10.2021 01:00



Biology, 01.10.2021 01:00


Physics, 01.10.2021 01:00

Physics, 01.10.2021 01:00


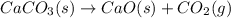
![\Delta G_{rxn}=\sum [n\times \Delta G_(product)]-\sum [n\times \Delta G_(reactant)]](/tpl/images/0659/5075/ad616.png)
![\Delta G_{rxn}=[(n_{CO_2}\times \Delta G_{CO_2})+(n_{CaO}\times \Delta G_{CaO})]-[(n_{CaCO_3}\times \Delta G_{CaCO_3})]](/tpl/images/0659/5075/3d6cf.png)
![\Delta G_{rxn}=[(1\times -394.4)+(1\times -604.17)]-[(1\times -1128.76)]](/tpl/images/0659/5075/691fa.png)


