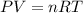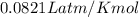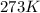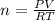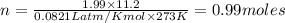Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

You know the right answer?
Which sample would have the same number of molecules as 11.2L of He (g) at 273K and 202kPa?
1...
1...
Questions

English, 14.07.2019 23:30

Mathematics, 14.07.2019 23:30




Chemistry, 14.07.2019 23:30


Mathematics, 14.07.2019 23:30


Chemistry, 14.07.2019 23:30


Mathematics, 14.07.2019 23:30




Chemistry, 14.07.2019 23:30

Mathematics, 14.07.2019 23:30



History, 14.07.2019 23:30

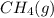 at 273K and 202kPa
at 273K and 202kPa