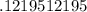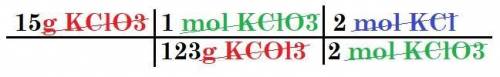Chemistry, 20.05.2020 23:57 whocares1234
Given the following reaction:
2 KCIO3
---> 2 KCl + 302
How many moles of KCl will be produced from 15,0 g of KCIO3?
moles

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2


Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 23.06.2019 06:30
(04.05 hc) analyze the given diagram of the carbon cycle below. an image of carbon cycle is shown. the sun, a cloud, two trees, one on the left and the other on the right, an animal, lake, and a factory are shown in the image. an arrow is shown from the sun towards the left tree marked a. the sun is marked b. there is an arrow from the air above the clouds, marked c, towards the left tree. an arrow from a location close to the ground marked d points towards dead organisms, which is a label under the animal. an arrow marked e points from the right tree straight up to the clouds. an arrow marked f points from the animal straight up to the clouds. an arrow marked g points from the factory towards the air above the clouds, c. there is an arrow pointing from the air to the lake labeled carbonates in water, an arrow pointing down from dead organisms to fossils and fossil fuels, and an arrow from fossils to the factory. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer.
Answers: 2
You know the right answer?
Given the following reaction:
2 KCIO3
---> 2 KCl + 302
How many moles of KCl will...
2 KCIO3
---> 2 KCl + 302
How many moles of KCl will...
Questions












History, 19.02.2021 18:20

Mathematics, 19.02.2021 18:20




Chemistry, 19.02.2021 18:20

SAT, 19.02.2021 18:20

Biology, 19.02.2021 18:20

Mathematics, 19.02.2021 18:20