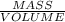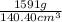Answers: 3
Another question on Chemistry

Chemistry, 20.06.2019 18:04
The volume of a sphere is given by v= (4/3) pi r cubed, where r is the radius. compute the volume of a sphere with a radius of 117pm. state your answer in units of cubed.
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
Ablock of lead weighed 1591 g and has dimensions 4.50cm by 5.20 cm by 6.00 cm calculate the density...
Questions


Mathematics, 27.12.2021 01:30

Mathematics, 27.12.2021 01:30

Physics, 27.12.2021 01:40




Mathematics, 27.12.2021 01:40

Physics, 27.12.2021 01:40

Spanish, 27.12.2021 01:40


Mathematics, 27.12.2021 01:50



Health, 27.12.2021 01:50

Mathematics, 27.12.2021 01:50

Mathematics, 27.12.2021 01:50


Physics, 27.12.2021 02:00

Social Studies, 27.12.2021 02:00