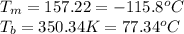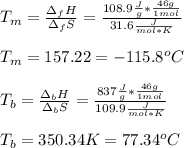Chemistry, 22.05.2020 01:10 questions61
For ethyl alcohol, C2H5OH, the enthalpy of fusion is 108.9 J/g, and the entropy of fusion is 31.6 J/mol •K. The enthalpy of vaporization at the boiling point is 837 J/g, and the molar entropy of vaporization is 109.9 J/mol •K.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

You know the right answer?
For ethyl alcohol, C2H5OH, the enthalpy of fusion is 108.9 J/g, and the entropy of fusion is 31.6 J/...
Questions


Health, 04.03.2021 08:10

Health, 04.03.2021 08:10

Mathematics, 04.03.2021 08:10


World Languages, 04.03.2021 08:10


Mathematics, 04.03.2021 08:10

Mathematics, 04.03.2021 08:10

Mathematics, 04.03.2021 08:10



History, 04.03.2021 08:10

Mathematics, 04.03.2021 08:10



Health, 04.03.2021 08:10

English, 04.03.2021 08:10

Mathematics, 04.03.2021 08:10