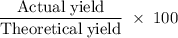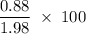Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

You know the right answer?
When 100.g Mg3N2 reacts with 75.0 g H2O, 15.0 g of NH3 is formed. What is the % yield?...
Questions

English, 18.05.2021 01:30


Arts, 18.05.2021 01:30


Mathematics, 18.05.2021 01:30

Chemistry, 18.05.2021 01:30

Mathematics, 18.05.2021 01:30


Chemistry, 18.05.2021 01:30

SAT, 18.05.2021 01:30




Arts, 18.05.2021 01:30

Mathematics, 18.05.2021 01:30



Mathematics, 18.05.2021 01:30


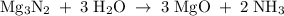
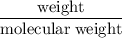
 =
=  mol
mol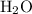 =
= 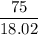
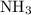 =
= 
 2 moles
2 moles