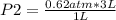Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
A gas has an initial volume of 3.0 L with a pressure of 0.62 atm. What will be the new pressure if t...
Questions

Social Studies, 16.01.2020 02:31

Computers and Technology, 16.01.2020 02:31


Chemistry, 16.01.2020 02:31


Spanish, 16.01.2020 02:31

English, 16.01.2020 02:31










Spanish, 16.01.2020 02:31


Biology, 16.01.2020 02:31