Chemistry, 22.05.2020 06:58 josephvcarter
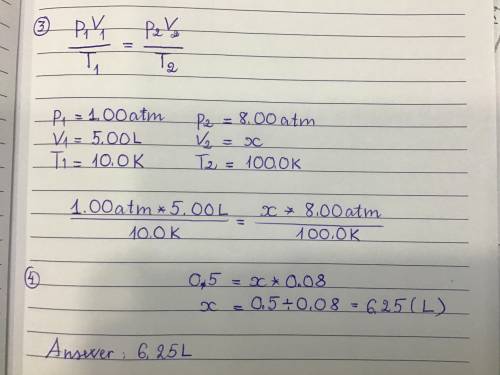
An ideal gas has a volume of 5.00L under a pressure of 1.00 atm and a temperature of 10.0 K. If the temperature is changed to 100.0 K while the pressure is increased to 8.00 atm what would be the new volume of the gas?
6.25L
1.60L
400.0L
16.0L

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Which of the following statements concerning the influence of culture on ethnic identity formation is accurate? a. one will reject ethnic identity if cultural stereotypes are encountered. b. if one’s ethnic city is different from the dominant cultural group, then one’s ethnic identity you will become weekend. c. if an the ethnic group is excepted by dominant culture, then ethnic identity formation can be a difficult process. d. similarity to the dominant culture can determine how easy it is for one to except ethnic differences.
Answers: 2

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
An ideal gas has a volume of 5.00L under a pressure of 1.00 atm and a temperature of 10.0 K. If the...
Questions




Mathematics, 10.02.2021 02:20



English, 10.02.2021 02:20



English, 10.02.2021 02:20




Mathematics, 10.02.2021 02:20

History, 10.02.2021 02:20

Biology, 10.02.2021 02:20

Mathematics, 10.02.2021 02:20


Biology, 10.02.2021 02:20

Mathematics, 10.02.2021 02:20