What would be the pH of an H2SO4 solution if the [H+] = 4.58 x 10-5 moles/liter?
9.66
4....

Chemistry, 22.05.2020 20:58 amelie1010
What would be the pH of an H2SO4 solution if the [H+] = 4.58 x 10-5 moles/liter?
9.66
4.34
5.67
NEXT QUESTION
3 ASK FOR HELP

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1
You know the right answer?
Questions


Mathematics, 21.05.2021 18:50

Chemistry, 21.05.2021 18:50


Mathematics, 21.05.2021 18:50

English, 21.05.2021 18:50





Mathematics, 21.05.2021 18:50

Advanced Placement (AP), 21.05.2021 18:50

Mathematics, 21.05.2021 18:50

Mathematics, 21.05.2021 18:50

Mathematics, 21.05.2021 18:50

SAT, 21.05.2021 18:50


Mathematics, 21.05.2021 18:50


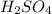 solution is 4.34
solution is 4.34![pH=-\log [H^+]](/tpl/images/0662/1654/37e81.png)
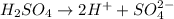
![pH=-\log[4.58\times 10^{-5}]](/tpl/images/0662/1654/0f1e2.png)
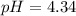
![[H^+]](/tpl/images/0662/1654/07acb.png) is
is ![4.58\times 10^{-5}]](/tpl/images/0662/1654/335c3.png) is 4.34
is 4.34

