of the solution?


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1


Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 10:00
Two moles of potassium chloride and three moles of oxygen are produced from the decomposition of two moles of potassium chlorate, kcos3(s). write the balanced equation. how many moles of oxygen are produced from 12 moles of potassium chlorate
Answers: 1
You know the right answer?
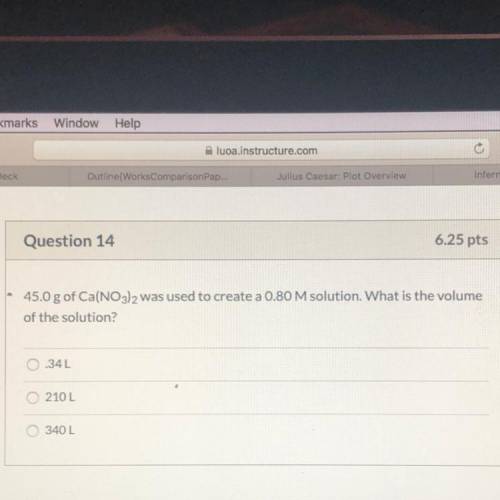
45.0 g of Ca(NO3)2 was used to create a 0.80 M solution. What is the volume
of the solution?
of the solution?
Questions

Physics, 09.12.2019 14:31


Mathematics, 09.12.2019 14:31




Business, 09.12.2019 14:31

English, 09.12.2019 14:31



History, 09.12.2019 14:31


Mathematics, 09.12.2019 14:31





Mathematics, 09.12.2019 14:31