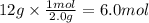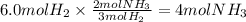1 point
Using the balanced chemical equation; how many moles of ammonia will
be made if...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 08:50
What happens after sound waves create vibrations in the ear?
Answers: 1
You know the right answer?
Questions





Arts, 10.06.2021 01:30

Advanced Placement (AP), 10.06.2021 01:30

Mathematics, 10.06.2021 01:30




Mathematics, 10.06.2021 01:30





Mathematics, 10.06.2021 01:30



Mathematics, 10.06.2021 01:30