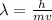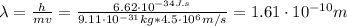Chemistry, 24.05.2020 03:59 Serenitybella
Use the de Broglie's Wave Equation to find the wavelength of an electron moving at 4.5 x 10^6 m/s. Please show your work. Note: h= Plank's constant (6.62607 x 10^-34 J s)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2



Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
Use the de Broglie's Wave Equation to find the wavelength of an electron moving at 4.5 x 10^6 m/s. P...
Questions

History, 05.03.2021 21:30

Mathematics, 05.03.2021 21:30




Mathematics, 05.03.2021 21:30


Mathematics, 05.03.2021 21:30


History, 05.03.2021 21:30

Health, 05.03.2021 21:30

History, 05.03.2021 21:30

Mathematics, 05.03.2021 21:30


Social Studies, 05.03.2021 21:30

Mathematics, 05.03.2021 21:30

Mathematics, 05.03.2021 21:30


French, 05.03.2021 21:30

History, 05.03.2021 21:30