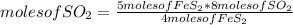Chemistry, 24.05.2020 04:02 briannagiddens
Calculate the moles of SO₂ produced when 5 moles of FeS₂ reacts according to the equation: 4FeS₂+ 11O₂ → 2Fe₂O₃ + 8SO₂

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which of the following is a compound? a.carbon b.oxygen c.hydrogen d.water
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 23.06.2019 04:10
What does the field of thermodynamics relate to a-changes in nuclear reactions b- changes in energy in systems c changes in molecular structure d changes in atomic properties
Answers: 1

Chemistry, 23.06.2019 12:30
17) large amounts of very important metal titanium are made by reacting magnesium metal with titanium tetrachloride. titanium metal and magnesium chloride are produced. a) write the balanced equation for this reaction. b) how many kilograms of magnesium are required to produce 1.00 kilograms of titanium? ( show work, .)
Answers: 1
You know the right answer?
Calculate the moles of SO₂ produced when 5 moles of FeS₂ reacts according to the equation: 4FeS₂+ 11...
Questions

Mathematics, 19.03.2021 20:10

Mathematics, 19.03.2021 20:10


History, 19.03.2021 20:10

English, 19.03.2021 20:10


English, 19.03.2021 20:10

Mathematics, 19.03.2021 20:10


Chemistry, 19.03.2021 20:10




Advanced Placement (AP), 19.03.2021 20:10

Mathematics, 19.03.2021 20:10



Mathematics, 19.03.2021 20:10