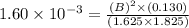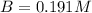At a certain temperature, the equilibrium constant for the chemical reaction shown is 1.60×10−3 . At equilibrium, the concentration of AB is 1.825 M, the concentration of BC is 1.625 M, and the concentration of AC is 0.130 M. Calculate the concentration of B at equilibrium. AB(aq)+BC(aq)↽−−⇀AC(aq)+2B(aq) AB ( aq ) + BC ( aq ) ↽ − − ⇀ AC ( aq ) + 2 B ( aq ) [B] =

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3
You know the right answer?
At a certain temperature, the equilibrium constant for the chemical reaction shown is 1.60×10−3 . At...
Questions



Mathematics, 21.03.2020 00:26

Mathematics, 21.03.2020 00:26



Mathematics, 21.03.2020 00:26

Mathematics, 21.03.2020 00:26


Mathematics, 21.03.2020 00:26





Mathematics, 21.03.2020 00:26



Social Studies, 21.03.2020 00:26


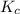
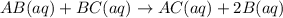
![K_c=\frac{[B]^2\times [AC]}{[BC]\times [AB]}](/tpl/images/0664/6603/29fb9.png)