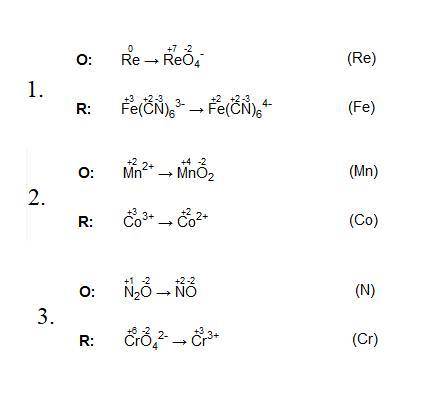
Fe(CN)63- + Re → Fe(CN)64- + ReO4--. What is reduced?
A. Fe
B. Re
Mn2+ + Co...


Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
Questions

Mathematics, 30.07.2019 16:30

History, 30.07.2019 16:30

English, 30.07.2019 16:30



History, 30.07.2019 16:30

SAT, 30.07.2019 16:30

Chemistry, 30.07.2019 16:30

English, 30.07.2019 16:30


World Languages, 30.07.2019 16:30

History, 30.07.2019 16:30

Social Studies, 30.07.2019 16:30

Mathematics, 30.07.2019 16:30



Health, 30.07.2019 16:30


History, 30.07.2019 16:30