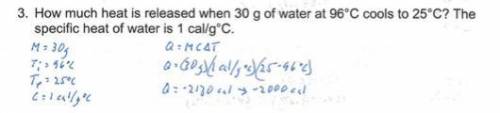Chemistry, 24.05.2020 01:02 tristanortonubca
3. How much heat is released when 30 g of water at 96°C cools to 25°C? The specific heat of water is
1cal/g°C.
ME
C=
AT=

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
3. How much heat is released when 30 g of water at 96°C cools to 25°C? The specific heat of water is...
Questions



Mathematics, 13.02.2020 18:40

Chemistry, 13.02.2020 18:40

Mathematics, 13.02.2020 18:40

Mathematics, 13.02.2020 18:40

Mathematics, 13.02.2020 18:40


Chemistry, 13.02.2020 18:40