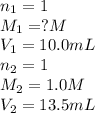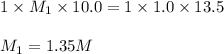Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
A 10.0 mL sample of HNO3 was exactly neutralized by 13.5 mL of 1.0 M KOH. What is the molarity of th...
Questions








English, 10.12.2020 02:30


Mathematics, 10.12.2020 02:30



Mathematics, 10.12.2020 02:30

Chemistry, 10.12.2020 02:30


Arts, 10.12.2020 02:30



Mathematics, 10.12.2020 02:30

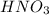 is 1.35 M
is 1.35 M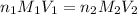
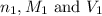 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 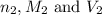 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.