- Given the following experimental data, use the method of initial
rates to determine the rate...

Chemistry, 26.05.2020 01:59 lydiadmanautou04
- Given the following experimental data, use the method of initial
rates to determine the rate law for the reaction aA + bB --> products.
Hint: Any number to the zero power equals one. For example,
(0.22) 1 and (55.6)° = 1.
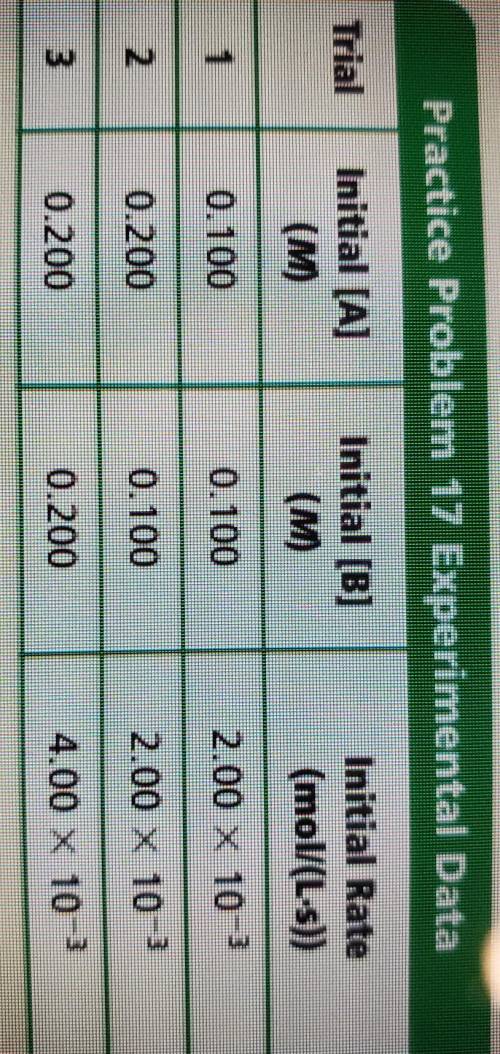

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 05:30
Scientist think that animals with remarkably similar embryo development probably shared a common ancestor
Answers: 1
You know the right answer?
Questions


Biology, 11.10.2019 09:30

Geography, 11.10.2019 09:30

Computers and Technology, 11.10.2019 09:30

Computers and Technology, 11.10.2019 09:30

Mathematics, 11.10.2019 09:30

English, 11.10.2019 09:30

History, 11.10.2019 09:30


Physics, 11.10.2019 09:30


Biology, 11.10.2019 09:30

Chemistry, 11.10.2019 09:30

History, 11.10.2019 09:30


Chemistry, 11.10.2019 09:30

Biology, 11.10.2019 09:30


Geography, 11.10.2019 09:30



