1. How many molecules of barium hydroxide, Ba(OH)2 are there in 21.9 grams of
barium hydroxide...

Chemistry, 26.05.2020 05:57 ayleenmorar
1. How many molecules of barium hydroxide, Ba(OH)2 are there in 21.9 grams of
barium hydroxide?
A. 6.022 x 1023 molecules Ba(OH)2
B. 7.7 x 1022 molecules Ba(OH)2
C. 6.23 x 1021 molecules Ba(OH)2
D. 5.93 x 1021 molecules Ba(OH)2

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1
You know the right answer?
Questions




English, 20.09.2020 08:01


Mathematics, 20.09.2020 08:01


Biology, 20.09.2020 08:01


Mathematics, 20.09.2020 08:01



Mathematics, 20.09.2020 08:01





Mathematics, 20.09.2020 08:01

Mathematics, 20.09.2020 08:01

Physics, 20.09.2020 08:01

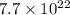 molecules of
molecules of 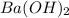
 of particles.
of particles.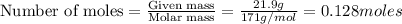
 molecules
molecules

