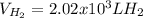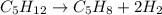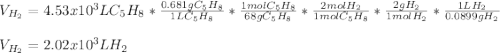Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3


Chemistry, 23.06.2019 21:30
25 points, the sap from a maple tree is an example of a concentrated solution weak solvent dilute solution suspension
Answers: 1
You know the right answer?
How many liters of H2 can form when 4.53 x 10^3 mL C5H8 form?...
Questions


English, 22.08.2019 03:00

Biology, 22.08.2019 03:00

Biology, 22.08.2019 03:00

Mathematics, 22.08.2019 03:00

Mathematics, 22.08.2019 03:00


Mathematics, 22.08.2019 03:00

Social Studies, 22.08.2019 03:00

Chemistry, 22.08.2019 03:00

Spanish, 22.08.2019 03:00

History, 22.08.2019 03:00

Physics, 22.08.2019 03:00

Mathematics, 22.08.2019 03:00


Mathematics, 22.08.2019 03:00

Mathematics, 22.08.2019 03:00

World Languages, 22.08.2019 03:00

Chemistry, 22.08.2019 03:00