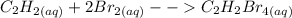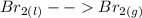Chemistry, 26.05.2020 09:58 jalenshayewilliams
equilibrium refers to a reaction in which the reactants and products are in the same phase; equilbrium refers to a reaction in which the reactants and products are in different phases.
A
Reversible; Non-Reversible
B
Heterogeneous; Homogeneous
C
Reversible; Heterogeneous
D
Homogeneous; Heterogeneous

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1
You know the right answer?
equilibrium refers to a reaction in which the reactants and products are in the same phase; equilbr...
Questions

Mathematics, 22.07.2019 14:00

Mathematics, 22.07.2019 14:00


Social Studies, 22.07.2019 14:00





Biology, 22.07.2019 14:00


History, 22.07.2019 14:00

Social Studies, 22.07.2019 14:00

Biology, 22.07.2019 14:00



Social Studies, 22.07.2019 14:00



Social Studies, 22.07.2019 14:00