
Chemistry, 27.05.2020 02:58 karinapenn8259
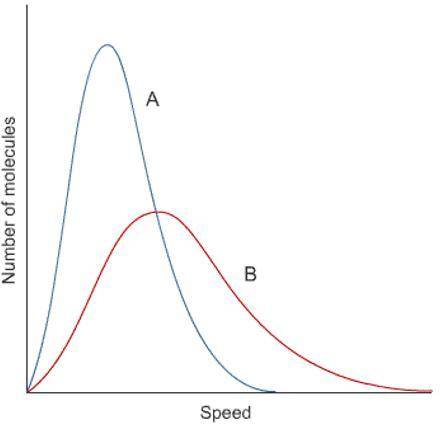
Using the image shown, select the statement that is true about the temperature of the gases and their kinetic energies. options:
A. Gas A is at a higher temperature because there are more molecules.
B Gas A is at a higher temperature because the molecules are moving faster. C. Gas B is at a higher temperature because there are more molecules.
D. Gas B is at a higher temperature because the molecules are moving faster.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Using the image shown, select the statement that is true about the temperature of the gases and thei...
Questions


History, 30.07.2019 05:00




Chemistry, 30.07.2019 05:00


Biology, 30.07.2019 05:00









Mathematics, 30.07.2019 05:00


Biology, 30.07.2019 05:00




