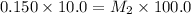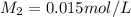1. 90.0 mL of distilled water is added to a 10.0mL sample of 0.150mol/L sodium
chloride. The r...

Chemistry, 27.05.2020 02:58 xaviiaquino3378
1. 90.0 mL of distilled water is added to a 10.0mL sample of 0.150mol/L sodium
chloride. The resulting solution can be described as more
a. dilute
b. concentrated
c. Acidic
d. basic

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
Questions

Mathematics, 06.05.2021 03:20


English, 06.05.2021 03:20



Mathematics, 06.05.2021 03:20



Mathematics, 06.05.2021 03:20

Mathematics, 06.05.2021 03:20

Mathematics, 06.05.2021 03:20

Mathematics, 06.05.2021 03:20

Mathematics, 06.05.2021 03:20

Arts, 06.05.2021 03:20

History, 06.05.2021 03:20



Mathematics, 06.05.2021 03:20

Mathematics, 06.05.2021 03:20

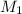 =concentration of stock solution = 0.150 mol/L
=concentration of stock solution = 0.150 mol/L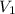 = volume of stock solution = 10.0 ml
= volume of stock solution = 10.0 ml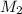 = concentration of dilute solution = ?
= concentration of dilute solution = ?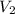 = volume of dilute solution = (10.0+90.0) ml = 100.0 ml
= volume of dilute solution = (10.0+90.0) ml = 100.0 ml