
Chemistry, 28.05.2020 02:58 cyclonenetwork1188
Consider the following system at equilibrium where H° = 10.4 kJ, and Kc = 1.80×10-2, at 698 K. 2HI(g) H2(g) + I2(g) When 0.40 moles of H2(g) are added to the equilibrium system at constant temperature: The value of Kc The value of Qc Kc. The reaction must run in the forward direction to restablish equilibrium. run in the reverse direction to restablish equilibrium. remain the same. It is already at equilibrium. The concentration of I2 will

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

You know the right answer?
Consider the following system at equilibrium where H° = 10.4 kJ, and Kc = 1.80×10-2, at 698 K. 2HI(g...
Questions



History, 11.12.2020 01:40


English, 11.12.2020 01:40

Mathematics, 11.12.2020 01:40




Mathematics, 11.12.2020 01:40

Business, 11.12.2020 01:40


Mathematics, 11.12.2020 01:40

Mathematics, 11.12.2020 01:40

Biology, 11.12.2020 01:40


English, 11.12.2020 01:40


Mathematics, 11.12.2020 01:40

Mathematics, 11.12.2020 01:40

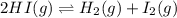
![Qc=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0668/1770/280b3.png)


