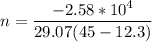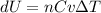Nitrogen gas in an expandable container is cooled from 45.0 C to 12.3 C with the pressure held constant at 2.58 ∗ 105 Pa. The total heat liberated by the gas is 2.58 ∗ 104 J. Assume that the gas may be treated as ideal. Find (a) the number of moles of gas; (b) the change in internal energy of the gas; (c) the work done by the gas. (d) How much heat woul

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2
You know the right answer?
Nitrogen gas in an expandable container is cooled from 45.0 C to 12.3 C with the pressure held const...
Questions






Chemistry, 12.01.2021 20:50



Mathematics, 12.01.2021 20:50

Mathematics, 12.01.2021 20:50

Biology, 12.01.2021 20:50

Mathematics, 12.01.2021 20:50

Mathematics, 12.01.2021 20:50

Mathematics, 12.01.2021 20:50



Mathematics, 12.01.2021 20:50

Mathematics, 12.01.2021 20:50


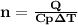
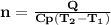
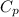 is the specific heat at constant pressure of Nitrogen gas which is = 29.07 J/mol/K
is the specific heat at constant pressure of Nitrogen gas which is = 29.07 J/mol/K