Chemistry, 28.05.2020 04:57 jacquetpaul1969
A key step in the extraction of iron from its ore is FeO(s) + CO(g) ⇋ Fe(s) + CO2(g) Kp =0.403 at 1000°C. What are the equilibrium partial pressures of carbon monoxide and carbon dioxide when 1.00 atm of carbon monoxide and excess iron(II) oxide react in a sealed container at 1000°C. write Kp expression, do ICE chart with pressures, 0.287 atm CO2 and 0.713 atm CO

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

You know the right answer?
A key step in the extraction of iron from its ore is FeO(s) + CO(g) ⇋ Fe(s) + CO2(g) Kp =0.403 at 10...
Questions




Mathematics, 16.12.2021 04:00


Mathematics, 16.12.2021 04:00





English, 16.12.2021 04:00

Mathematics, 16.12.2021 04:00



Health, 16.12.2021 04:00



Social Studies, 16.12.2021 04:00

English, 16.12.2021 04:00

Chemistry, 16.12.2021 04:00

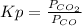 = 0.403
= 0.403