
Chemistry, 28.05.2020 03:57 arianawelsh123l
"A gas turbine receives a mixture having the following molar analysis: 10% CO2, 19% H2O, 71% N2, at 720 K, 0.35 MPa and a volumetric flow rate of 3.2 m3 /s. The mixture exits the turbine at 380 K, 0.11 MPa. For adiabatic operation with negligible kinetic and potential energy effects, determine the power developed at steady state, in kW." NOTE: the process is NOT isentropic.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1
You know the right answer?
"A gas turbine receives a mixture having the following molar analysis: 10% CO2, 19% H2O, 71% N2, at...
Questions










Mathematics, 17.12.2021 04:30




Mathematics, 17.12.2021 04:30

Mathematics, 17.12.2021 04:30

Mathematics, 17.12.2021 04:30


History, 17.12.2021 04:30


Mathematics, 17.12.2021 04:30

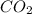 =0.1
=0.1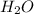 = O.19
= O.19
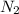 =0.71
=0.71
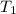 ) mixture receives from turbine =720K
) mixture receives from turbine =720K
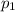 ) mixture receives from turbine =0.35 Mpa
) mixture receives from turbine =0.35 Mpa
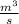
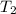 ) = 380K
) = 380K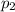 )= 0.11 Mpa
)= 0.11 Mpa
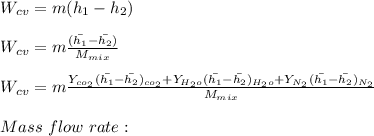
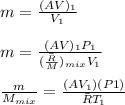
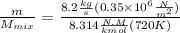
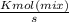
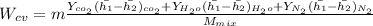
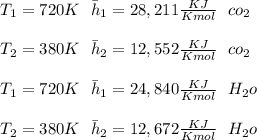
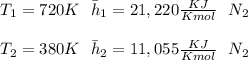
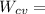 2074.2 KW
2074.2 KW

