
Chemistry, 27.05.2020 19:01 laneycasey9058
One liter of oxygen gas at standard temperature and pressure has a mass of 1.43 g. The same volume of hydrogen gas under these conditions is 0.089 g. If both volumes contain the same number of gas particles (according to Avogadro's hypothesis), how can this difference in mass be explained?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
One liter of oxygen gas at standard temperature and pressure has a mass of 1.43 g. The same volume o...
Questions

History, 19.07.2019 20:00

Biology, 19.07.2019 20:00


Mathematics, 19.07.2019 20:00

Mathematics, 19.07.2019 20:00

English, 19.07.2019 20:00


Mathematics, 19.07.2019 20:00

Mathematics, 19.07.2019 20:00

Arts, 19.07.2019 20:00

Biology, 19.07.2019 20:00

English, 19.07.2019 20:00

Biology, 19.07.2019 20:00

Biology, 19.07.2019 20:00

Biology, 19.07.2019 20:00

Health, 19.07.2019 20:00

Biology, 19.07.2019 20:00

Health, 19.07.2019 20:00

Computers and Technology, 19.07.2019 20:00

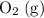 is larger than that of
is larger than that of 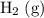 (by a factor of about
(by a factor of about 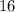 .) Therefore, the mass of the
.) Therefore, the mass of the 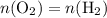 .
.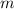 is different from the number of gas particles
is different from the number of gas particles 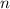 in it. In particular, if all particles in this gas have a molar mass of
in it. In particular, if all particles in this gas have a molar mass of 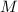 , then:
, then: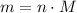 .
.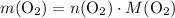 .
.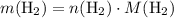 .
.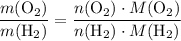 .
.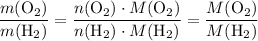 .
. :
: 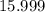 .
.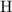 :
: 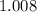 .
.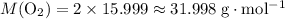 .
.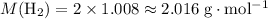 .
.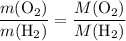 :
: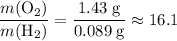 .Right-hand side:
.Right-hand side: 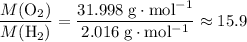 .
.

