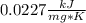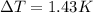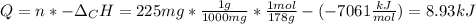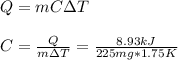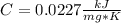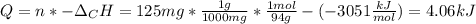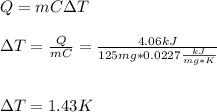Chemistry, 28.05.2020 18:57 robert7248
When 225mg of anthracene, C14H10(s), was burned in a bomb calorimeter the temperature rose by 1.75K. Calculate the calorimeter constant. By how much will the temperature rise when 125mg of phenol, C6H5OH(s), is burned in the calorimeter under the same conditions? (ΔcH<(C14H10,s)=–7061 kJ mol−1.)

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1
You know the right answer?
When 225mg of anthracene, C14H10(s), was burned in a bomb calorimeter the temperature rose by 1.75K....
Questions

Mathematics, 13.11.2020 23:20


Geography, 13.11.2020 23:20

English, 13.11.2020 23:20

Mathematics, 13.11.2020 23:20

English, 13.11.2020 23:20


Mathematics, 13.11.2020 23:20


Mathematics, 13.11.2020 23:20


Advanced Placement (AP), 13.11.2020 23:20

Mathematics, 13.11.2020 23:20

Chemistry, 13.11.2020 23:20

English, 13.11.2020 23:20


Mathematics, 13.11.2020 23:20