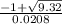Chemistry, 29.05.2020 00:00 putaprincess16
A container has a mixture of NO2 gas and N2O4 gas in equilibrium. The chemical reaction between the two gases is described by the equation 2NO2 ⇌ N2O4. The partial pressure of NO2 is 61.2 MPa and the partial pressure of N2O4 is 38.8 MPa. Several weights are added to the lid of the container, increasing the total pressure on the container to 200 MPa. What are the most likely partial pressures when equilibrium is established again?3.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
A container has a mixture of NO2 gas and N2O4 gas in equilibrium. The chemical reaction between the...
Questions

Chemistry, 03.07.2019 22:00



History, 03.07.2019 22:00

Mathematics, 03.07.2019 22:00

History, 03.07.2019 22:00

English, 03.07.2019 22:00

Social Studies, 03.07.2019 22:00

Mathematics, 03.07.2019 22:00

Mathematics, 03.07.2019 22:00



Biology, 03.07.2019 22:00

Physics, 03.07.2019 22:00


Computers and Technology, 03.07.2019 22:10

Computers and Technology, 03.07.2019 22:10

Computers and Technology, 03.07.2019 22:10

Mathematics, 03.07.2019 22:10

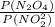
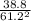
![\frac{200 - P(NO_{2}) }{[P(NO_{2} )]^{2}}](/tpl/images/0669/4562/5f3ab.png)
![[P(NO_{2} )]^{2}](/tpl/images/0669/4562/76f80.png) +
+ 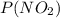 - 200 = 0
- 200 = 0