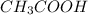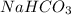Chemistry, 28.05.2020 15:00 stefancvorovic1
During an experiment vinegar was added to baking soda. The following observations were recorded.
Observation 1: The container with the reaction mixture feels cooler.
Observation 2: Gas bubbles are released.
Based on the observation, justify the type of change (physical or chemical) that took place.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:10
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1


Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

You know the right answer?
During an experiment vinegar was added to baking soda. The following observations were recorded.
Questions

Mathematics, 04.03.2020 05:31







Mathematics, 04.03.2020 05:32


Mathematics, 04.03.2020 05:32


Social Studies, 04.03.2020 05:32




Mathematics, 04.03.2020 05:32